- Home>Products Display>Alkoxy silanes > Methyltrimethoxysilane
Product Categories
Silicon Technology
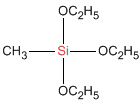
Methyltrimethoxysilane
-
CAS No.
1185-55-3
-
Molecular Formula :
C4H12O3Si
-
Purity :
98%
-
Annual capacity :
1000 Ton per year
-
Technical Index :
Density(ρ20, g/cm3): 0.9500 ± 0.0050
Refractive Index(n25D): 1.3695 ± 0.0050 -
Packing :
210LIron Drum:180kg/drum
1000L IBCContainer: 900kg/container
Description :
Methyltrimethoxysilane is highly miscible with standard organic solvents,such as alcohols, hydrocarbons and acetone. Methyltrimethoxysilane is practically insoluble in neutral water and reacts only slowly to form silanols and higher condensation products. Addition of a hydrolytic catalyst (inorganic/organic acids, ammonia or amines) accelerates the hydrolysis of Methyltrimethoxysilane substantially. (Filler Modifier) Methyltrimethoxysilane is used mainly to render a wide range of surfaces and materials water repellent (e.g.mineral fillers,pigment,glass cardboard). Methyltrimethoxysilane may be used pure or in solution to treat fillers, using suitable mixing equipment.It may be necessary to first pre-treat the substrate with water and/or a catalyst. Methyltrimethoxysilane is also used in the production of silicone resins and condensation-curing silicone rubber.

Operation Instruction of IBC :
Inner layer: 4-fluoroethylene drum, 250 kg volume 220kg net weight, and other Argon Protective Layers
Middle layer: 6-7 layers of foam for earthquake Prevention
Outer layer : Fixation of steel frame or hard plastic shell and bottom silica gel protection
Methyltrimethoxysilane is an organosilicon compound with the formula CH3Si(OCH3)3. It is a colorless, free-flowing liquid. It is a crosslinker in the preparation of polysiloxane polymers.
Methyltrimethoxysilane Preparation, structure and reactivity
Methyltrimethoxysilane is usually prepared from methyltrichlorosilane and methanol:
CH3SiCl3 + 3 CH3OH → CH3Si(OCH3)3 + 3 HCl
Alcoholysis of alkylchlorosilanes typically proceeds via an SN2 mechanism. Inversion of the configuration is favored during nucleophilic attack when displacing good leaving groups, such as chloride. In contrast, displacement of poor leaving groups, such as alkoxide, retention is favored.
Methyltrimethoxysilane is tetrahedral and is often described as sp3 hybridized. It has idealized C3v point symmetry.
Hydrolysis of MTM proceeds both under acidic and basic conditions. Under acid conditions, rates of successive hydrolyses for methyltrimethoxysilane decreases with each step. Under basic condition the opposite is true.